
April 26, 2021--- Scivita Medical Technology Co., Ltd. ("Scivita Medical") today announced that it has raised nearly RMB0.4 Billion in Series A funding round, which has become one of the highest financing projects in the field of endoscope in China in recent years. The investment was jointly led by GL Ventures and Lilly Asia Ventures, with contribution from Matrix Partners China, Medtronic, Chengwei Capital and Shanghai Innochip Investment. The successful completion of the Series A funding round reflects the recognition of Scivita Medical in the field of endoscope and the expectation of its future development from the capital market and industrial strategic partners. Scivita Medical will strive to build the world's leading platform for innovative products in endoscope and related fields.
Founded by Dr. ZHANG Yi and Dr. CHEN Dong, Scivita Medical is headquartered in Suzhou Industrial Park with R&D centers in Suzhou, China, and Tokyo, Japan, focusing on the image processing R&D and technology innovation of endoscope. Scivita Medical is a high-tech enterprise engaged in the field of optical diagnosis and treatment, multidimensional images development, high-performance materials research and precision manufacturing with minimally invasive medical treatment. Scivita Medical's product covers both flexible endoscopy and rigid endoscopy, and has unique advantages in the research and commercialization of all cutting-edge technologies in the field of medical endoscopes. It has ultra-high-definition visualization technology (such as 4K UHD visualization), 3D visualization technology, special light visualization technology (such as fluorescence visualization), ultra-fine endoscopic visualization technology, single-use technology, etc.
According to Frost & Sullivan, it is estimated that by 2024, the overall scale of the global endoscope devices market will grow to USD26.98 billion, and the Chinese endoscope market size will reach RMB42.3 billion, becoming one of the fastest growing medical device sub-industries in the world. With the popularization of minimally invasive diagnosis and treatment technology worldwide, the endoscope industry has also entered a stage of rapid development.
The endoscope industry has higher technical barriers, involving multi-disciplinary and multi-fields, which integrates medicine, optics, materials, precision machinery, electronic engineering, software development and other disciplines, and has more technical content as compared to traditional mechanical manufacturing. At present, the endoscope equipment market presents an oligopolistic situation, with international brands occupying a large market share by relying on first-mover advantages and mature technological advantages. Scivita Medical has successfully achieved breakthroughs in key areas such as optical imaging and image processing of endoscopes, breaking down the technological monopoly of international brand products.
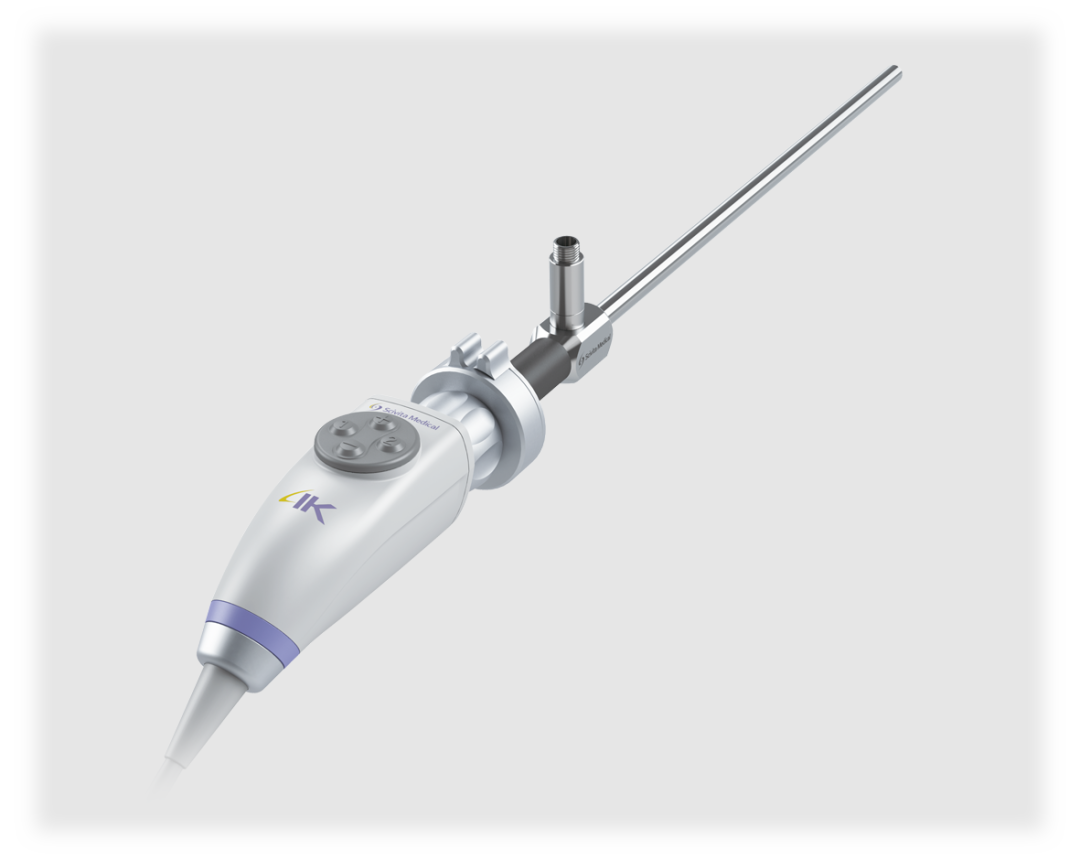
Scivita Medical accurately grasps the clinical needs, and combines innovative technologies in multi-disciplinary fields to develop excellent Chinese products that are recognized by clinical experts worldwide.World's first 2D to 3D " 3D Visualization System" developed by Scivita Medical obtained NMPA certification and CE certification in July 2018, and became the first domestic 3D Visualization System approved by the U.S. FDA launched in the United States in September 2019. The "4K UHD Camera System" independently developed by Scivita Medical obtained NMPA certification in June 2019. It was launched in China as the first domestic 4K UHD camera system and obtained CE certification in July 2019. In August 2020, the "4K UHD Camera System" became the first Chinese 4K product that was certified by the U.S. FDA.
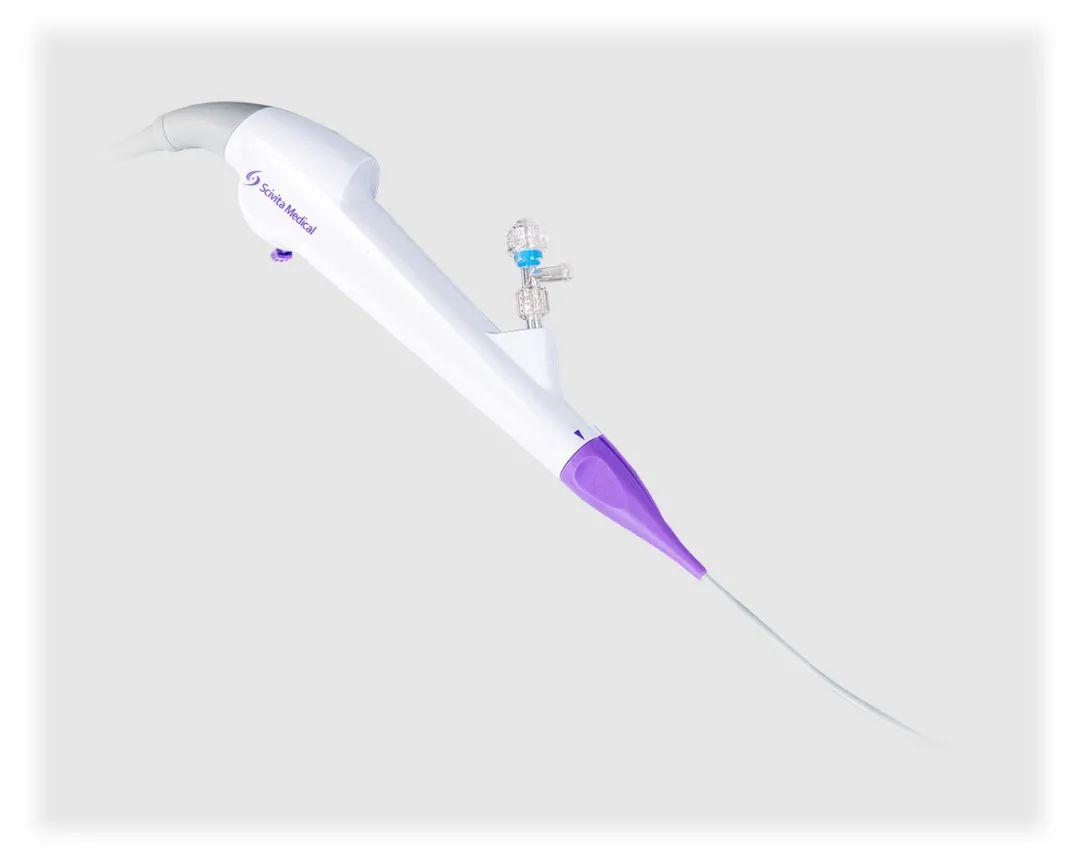
In addition, Scivita Medical has various single-use endoscopy devices with international competitiveness, many of which have entered the clinical, registration, or sales stages in China, the United States, Europe and other countries and regions, respectively. Given that endoscope industry has entered into the era of consumables, Scivita Medical will compete with international brands in the global market with its disruptive single-use technology and leading technological advantages.
Dr. ZHANG Yi, Co-founder of Scivita Medical, said: "The company's founding management team expresses sincere gratitude to the industry's top investment institutions and industry strategic partners for their recognition and support. In the next few years, endoscope products will fully realize the iteration from 'HD' to 'Ultra HD', and from 'reuse' to 'single-use'. Relying on the world's leading technology and strong sustainable R&D strength, and with the support from investment institutions and the continuous attention from our peers, Scivita Medical will form a platform with a comprehensive layout of innovative products in endoscope and related fields. It is committed to using domestic high-quality innovative products to replace and compete with international brand products, and striving to build a world-leading endoscope R&D brand that are rooted in China. The original intention of Scivita Medical is to solve the clinical pain points, making diagnosis and treatment safer and more effective, and help every patient and their family. Scivita Medical will make unremitting efforts for it. "
Michael Nuoqing YI, Co-Chief Investment Officer of Hillhouse and Head of Biomedicine and Medical Devices of GL ventures, said: "With the advancement of hierarchical diagnosis and treatment, the development of minimally invasive surgery and the aging population, the clinical demand for endoscope is rising rapidly and there is a huge room for domestic substitute. Scivita Medical takes a leading position at home and abroad. Its products are independently developed, which covers electronic and optical endoscopes. It has ultra-high-definition visualization technology, 3D visualization technology, special light visualization technology, ultra-fine endoscopic visualization technology and single-use technology. Hillhouse would like to have a long-term companionship with outstanding entrepreneurs like Dr. ZHANG Yi and Dr. CHEN Dong, who are innovative and with excellent management experience. Hillhouse also aims to help Scivita Medical continue to make efforts on R&D and innovation, so as to using domestic high-quality innovative products to replace international brand products, and actively participate in international competition and to benefit more patients."
Dr. CHEN Fei, Managing partner of Lilly Asia Ventures, said: "The technical barrier of endoscope industry is very high. We are very optimistic about the strong technical experience and innovative R&D capabilities of Scivita Medical team in terms of rigid and flexible endoscopes. The 4K UHD Camera System independently developed by Scivita Medical was the first one approved by China National Medical Products Administration and FDA, and it is highly recognized by clinical experts. Leverage on its distinctive product solutions and forward-looking industrial layout, we believe Scivita Medical can become the leader in technological innovation in the field of endoscopy in China and even in the world.
Dr. SHI Yonghui, Managing Director of the Corporate Development and Venture Capital Department (Greater China) of Medtronic, said: "It is Medtronic's global mission to relieve pain, restore health, and extend life for patients around the world. Medtronic China has always been committed to supporting the application of innovative medical technology in the local market. Since the establishment of the Venture Capital Fund and Innovation Accelerator after 2016, it has invested in and incubated more than 10 medical technology companies. By strategically investing in Scivita Medical, we hope that both parties could carry out multi-level strategic cooperation in the field of endoscope, and finally bring prime medical solutions to thousands of Chinese patients."
Source: Scivita Medical